HG Energy researchers co-author Nature Catalysis study on solar-driven methane conversion
Jan, 23, 2026
A new study published in Nature Catalysis showcases a promising strategy for methane conversion using solar energy, with the participation of researchers from HG Energy in collaboration with teams from the China University of Geosciences (Wuhan) and the University of Science and Technology of China (Hefei).
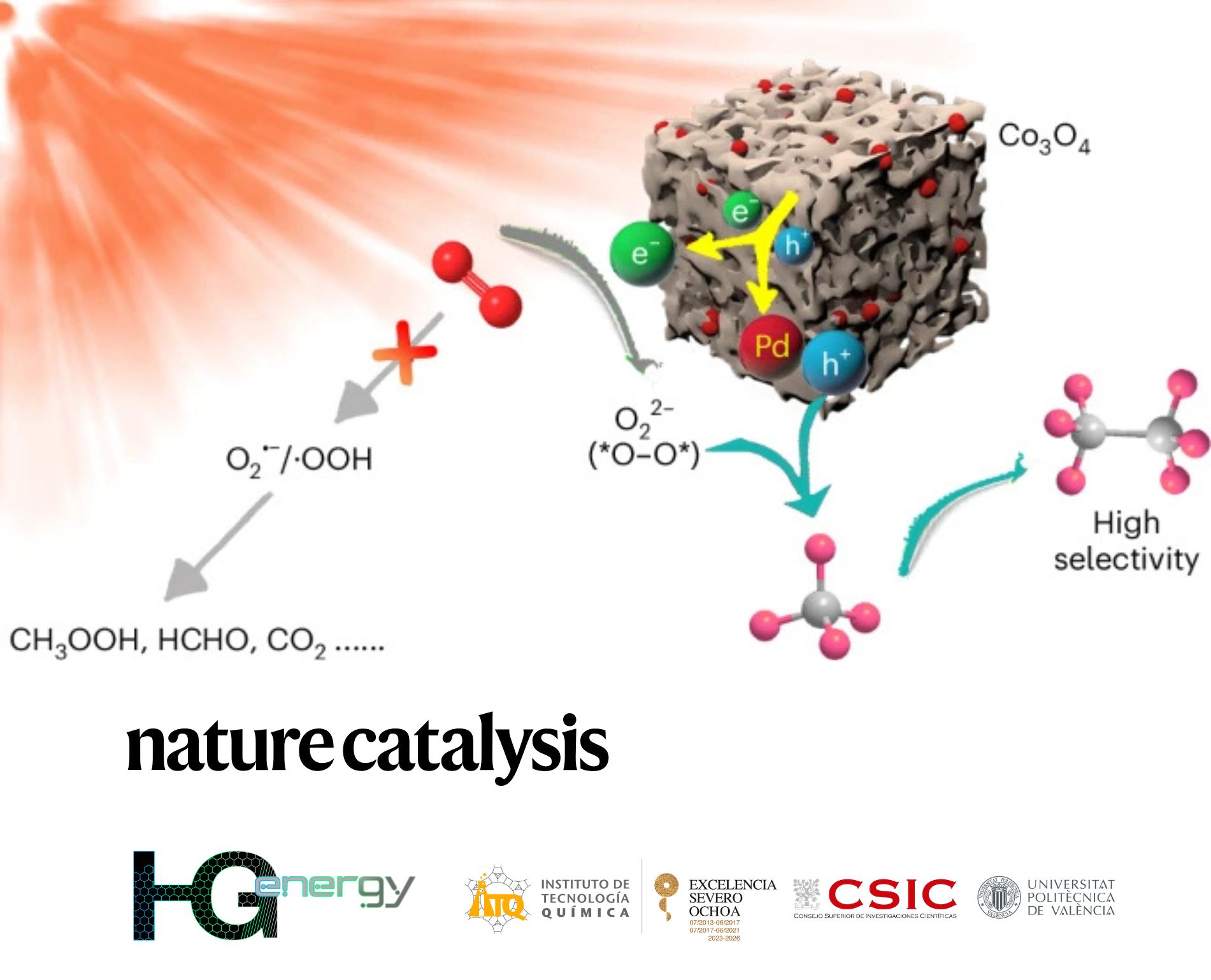
Titled “Co₃O₄ as full-solar-spectrum photocatalyst for selective methane conversion through reactive oxygen species control”, the work presents a novel photocatalyst based on cobalt oxide (Co₃O₄) combined with palladium. This material absorbs across the full solar spectrum and selectively transforms methane into ethane (C₂H₆), avoiding overoxidation and minimizing by-products such as CO₂ or methanol.
The process addresses one of the major challenges in methane utilization: its chemical inertness. The catalyst developed by the research team achieves a selectivity of 96.2% for ethane and a conversion rate of 16.1 mmol·g⁻¹·h⁻¹ under ambient conditions.
Researchers from HG Energy contributed to understanding the reaction mechanism at the molecular level, particularly how charge carriers and reactive intermediates behave at the catalyst interface. Their insights help explain how the system balances high activity with remarkable selectivity, paving the way for more efficient solar-driven chemical processes.
This international collaboration demonstrates the value of combining expertise in catalysis, materials science and photochemistry to tackle key energy challenges.
The full article is available at: https://doi.org/10.1038/s41929-025-01471-x